
Core Protein
HBc antigen
For most of genotypes, HBc antigen contains 183 or 185 amino acids (aa), but HBc of genotype G has an additional internal sequence which makes it longer (195 aa). The N-terminal assembly domain (NTD) is involved in core particles assembly (Figure 1) and the C-terminal functional domain (arginine-rich) is involved in packaging of the pregenome/reverse transcriptase complex [1].
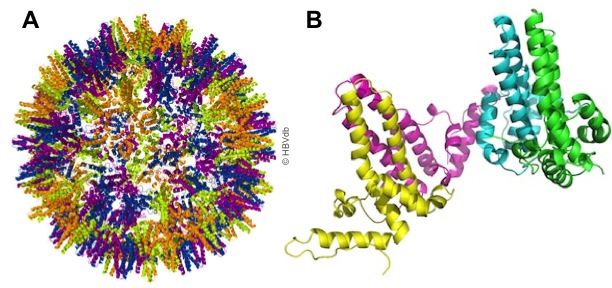 |
| Figure 1. Core protein structure. PDB:1qgt [2]. (A) The core assembly in capsid (model of 120 dimers). (B) The structure of core subunit (T=4, chain A,B,C,D). Coloration by chain. |
HBe antigen (External core antigen)
HBe protein is translated from the first ATG of C ORF, and thus contains a N terminal extension of 29 amino acids. This is an hydrophobic region which forms a signal peptide which directs the protein to the endoplasmic reticulum (ER). Then, the protein transits through the Golgi to the cellular surface. During the transport, the signal peptide and the basal tail are removed. The mature protein, HBeAg, is secreted as a monomeric protein, which plays a role in the immune system escape [3].
Dataset
Bibliographic references
Hepatitis B virus replication.
Beck J., Nassal M.
World J Gastroenterol, 2007, 13(1):48-64.The crystal structure of the human hepatitis B virus capsid.
Wynne S.A., Crowther R.A., Leslie A.G.
Mol Cell, 1999, 3(6):771-780.Envelopment of the hepatitis B virus nucleocapsid.
Bruss V.
Virus Res, 2004, 106(2):199-209.